It has been estimated that up to one quarter of the world’s biomass is of fungal origin, comprising approximately 6 million species. In order to interact with one another and respond to environmental cues, these species communicate via chemical languages using extracellular signals and cellular responses. In filamentous ascomycete fungi, the formation of an interconnected, multinucleate, syncytial network is constructed via hyphal fusion (anastomosis). Anastomosis in filamentous fungi is comparable to somatic cell fusion events resulting in syncytia in other eukaryotic organisms, including myoblast fusion during muscle differentiation, fusion between macrophages in the formation of osteoclasts and trophoblast fusion during placental development in mammals.
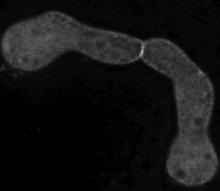
 Cell fusion of germinated spores of Neurospora crassa. (Glass et al, 2004)
Cell fusion of germinated spores of Neurospora crassa. (Glass et al, 2004)
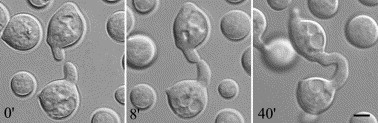
Cell fusion between genetically identical cells (self-fusion) is essential for the normal development of most multicellular eukaryotes. Despite its importance, the molecular basis of self-fusion is poorly understood. In Neurospora crassa, germinating vegetative cells chemotropically sense genetically identical partner cells at a distance and undergo mutual recognition-directed growth and ultimately cell fusion. Hyphae in the interior of mature colonies also undergo recognition and fusion, in order to maintain interconnected hyphal networks.
Understanding the molecular basis of germling and hyphal fusion provides a paradigm for self-signaling mechanisms in eukaryotic microbial species, and may also provide a useful model for somatic cell fusion events in other eukaryotic species. Directing growth along a chemoattractant gradient is a common cellular mechanism that requires functional differences between the communicating cells, with one cell acting as the signal sender and the other as the signal receiver. During self-fusion, it is unclear exactly how these functional differences arise.
We have characterized a number of hyphal and germling fusion mutants in N. crassa, including a MAP kinase signal transduction pathway that is essential for the initiation of fusion (Pandey et al., 2004). In addition, we identified a WW protein, SOFT (Fleissner et al., 2005) and a putative transmembrane protein, HAM-2 (Xiang et al., 2002), both of which are required for chemotropic interactions. We have recently shown that communicating cells prior to self-fusion rapidly alternate between two physiological states that may be associated with signal delivery and response pathways (Fleissner et al., 2009). These states can bemarked by the localization of MAK-2 and SO, which are alternately recruited in both cells of a communicating pair, in opposition. This is shown in the timelapse movies and model (See the Gallery pages! or use these links for the movies: Oscillation of MAK-2-GFP during communication and fusion,Oscillation of SO-GFP during communication and fusion, Oscillation of MAk-2-GFP and SO-dsRED during communication and fusion (Click on view detail and then SM1, SM2 and SM3 to start the movies) (from Fleissner et al., 2009- please see PNAS.ORG for the full paper and additional data on this signaling mechanism).
Using this system, pairs of genetically identical cells coordinate their behavior over time and space, enabling them to achieve mutual recognition and directed growth while avoiding self-stimulation, and ultimately undergo vegetative cell fusion.
Our current research objectives are to identify the receptor and ligands associated with self signaling and to understand how chemotropic interactions result in polarization of cytoskeletal elements.